Base Editing Systems: Promise and Shortcomings
The development of CRISPR base editing systems was a game-changer in the gene editing field. These derivatives of CRISPR-Cas9 technology can induce point mutations at target sites in the genome with high efficiency, correcting or disrupting genes without the need for creating double-stranded breaks. In addition, base editors do not need donor DNA templates for repair, making them even simpler to use.
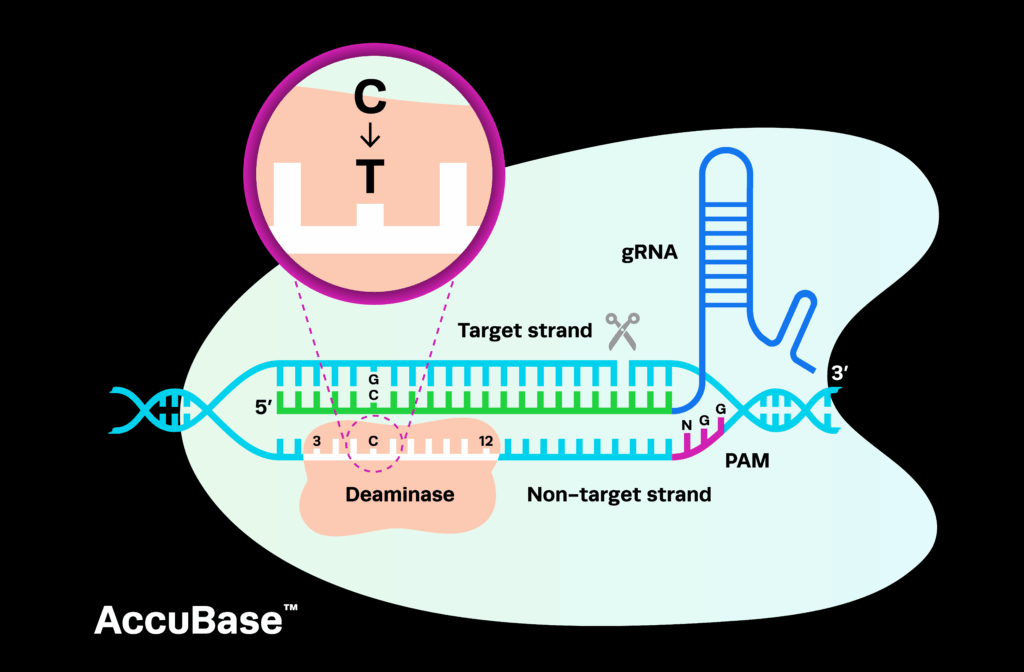
Base editors use three components: a deaminase enzyme, which induces the mutations; a single guide RNA (sgRNA), which directs the enzymes to the target site; and a Cas9 nickase (nCas9) or catalytically dead Cas9 (dCas9), which locates the target site and, in the case of nCas9, nicks the top strand of DNA. Because they rely on a Cas enzyme to direct the editing activity, they are restricted to targets with an appropriately located protospacer adjacent motif (PAM) site.
There are two key types of base editing systems: cytosine base editors (CBEs) and adenine base editors (ABEs). CBEs employ a naturally occurring cytosine deaminase enzyme, such as APOBEC, to induce C•G to T•A base pair conversions, while ABEs use an engineered adenosine deaminase, like TadA, to induce A•T to G•C mutations.
Around half of the known genetic variants that cause disease in humans are caused by single-nucleotide variants (SNVs), making base editors an attractive approach for therapeutic gene editing. However, base editors are not without their flaws; they can induce substantial off-target edits in DNA and RNA, resulting in undesirable effects in the genome and transcriptome. This includes bystander edits, which occur at bases near the target within the editing window.
Concerns around the off-target activity of base editors have been a major obstacle to their clinical translation, prompting researchers to explore methods to reduce this activity.
New to Base Editing? Start Here
Not sure where to begin in your base editing workflow? In our webinar, we break it down step-by-step from gRNA design to analysis, making it easy to follow and apply!
The Rationale Behind Cas-Embedded Base Editors
Before we delve into how AccuBase can be used to pursue clinically relevant targets, let’s explore how the team at Kactus generated this unique base editing system, as described in their 2020 Nature publication. The study focuses on why the authors postulated that embedding base editing enzymes in nCas9 would reduce off-targets, a screen to identify tolerant sites for insertion in nCas9, and testing in human and mouse cells to determine the on-target and off-target profiles of these Cas-embedded systems. The team began with ABEs as proof-of-concept, then used the same approach for CBEs.
Why embed deaminase enzymes?
Several research groups have attempted to create base editors with minimal off-target activity by mutating the enzymes and/or inducing rationally engineered point mutations or deletions in the deaminases. However, these strategies can have other effects on the enzymes and are not always successful.
Previous studies have shown that when some base editing enzymes are expressed in human cells as free proteins, they produce more off-target effects than when they are tethered to the N terminus of nCas9. This finding led the team at Kactus to postulate that off-target activity could be reduced further by embedding the base editing enzymes: inserting them right into the middle of nCas9.

Screening for tolerable insertion sites
The first step was to perform a genetic screen to identify sites within nCas9 that would be tolerant of deaminase insertions without affecting the other functions of the protein. To do so, the team constructed a plasmid expressing several components:
- nCas9 under the control of an IPTG-inducible promoter
- The ampicillin resistance (AmpR) gene, bearing a C > T mutation that creates a premature stop codon
- An sgRNA under the control of the J23119 promoter, for repairing the premature stop codon
Any bacteria that have been transformed with this plasmid are sensitive to ampicillin until the stop codon is repaired, restoring translation. The authors then used a DNA transposon system to randomly insert the DNA sequence for the TadA-TadA* deaminase into this all-in-one plasmid, creating a library of plasmids with different TadA-TadA* insertion sites.
The plasmid was electroporated into Escherichia coli cells and the transformed cells were grown without ampicillin. Deep-sequencing of the plasmids from recovered cells revealed over 51,000 insertions within the nCas9 coding sequence, with insertions across 99% of amino acid positions.
IPTG was then added to induce the expression of nCas9-TadA-TadA* fusion proteins, and the cells were transferred to ampicillin plates, allowing the authors to select cells that expressed the repaired AmpR gene. Plasmids were extracted from the positive clones and sequenced to assess both insertion sites and editing efficiencies.
This approach yielded 43 tolerable insertion sites on nCas9 and the nCas9-TadA-TadA* fusion proteins were named Cas-embedded adenine base editors (CE-ABEs). Nine of the insertion sites were located in a 16-amino acid stretch located in the RuvC III domain in the NUC lobe of nCas9. This region is not conserved across SpCas9 orthologs, and appears to be highly tolerant of deaminase insertion while allowing nCas9 to function normally.
Testing in HEK293 cells
The authors next put the 20 most frequently recovered CE-ABEs to the test in HEK293T cells, comparing their performance to the ABEmax system. The target for editing was an endogenous site in the genome, in an editing window containing multiple As. These 20 tested CE-ABEs had editing efficiencies ranging from 66-89% - consistent with their editing efficiencies in E. coli - and 12 displayed the same level of activity as ABEmax.
Off-target RNA editing analysis showed that all 12 CE-ABEs had significant reductions in editing at off-target RNA sites that were highly subject to editing by ABEmax. The four top-performing CE-ABEs were also subjected to further testing and comparison, including RNA sequencing of off-target editing across the entire transcriptome. While ABEmax displayed an immense off-target editing profile, CE-ABEs had at least a six-fold reduction in this activity.
After confirming the on-target editing efficiency of CE-ABEs, the authors selected one variant, CE1048-1063-ABE, as having the ideal balance of specificity and efficiency. To further characterize its performance, the team tested it at several randomly selected sites in both human and mouse cells; it had a slightly enlarged editing window but displayed comparable editing efficiencies to ABEmax.
Embedding cytosine base editors with different APOBEC enzymes
Given the success of CE-ABEs, the team at Kactus wanted to apply the same embedding strategy to CBE systems. They did so with two CBEs that utilize different APOBEC enzymes: AncBE4max, a highly active but non-specific system that employs the APOBEC ancestor Anc689; and BE4-A3A, a highly active system that induces significant off-target editing in DNA, utilizing APOBEC3A. The latter system was previously manipulated by the authors in an attempt to minimize off-target editing, with limited success.
After embedding the APOBEC enzymes into the tolerant region of nCas9, the authors explored the effects on on-target editing at nine randomly selected targets in HEK293T cells. The Cas-embedded version of AncBE4max, CE1048–1063-CBE, retained the same levels of on-target editing activity. Similar results were seen with the Cas-embedded version of BE4-maxA3A(Y130F), CE1048–1063-A3A(Y130F).
Because BE-A3A systems are able to perform efficient editing in GC-rich and methylated regions of the genome, the authors also wanted to determine if the embedded CE1048–1063-A3A(Y130F) retained this advantage, assessing its activity at three hypermethylated target genes. CE1048–1063-A3A(Y130F) not only retained its ability to edit methylated areas, but outperformed its competitor, the highly active YE1-BE4max system.
The final step was to determine the off-target effects of the CE-CBEs in the genome and transcriptome. To do so, the authors performed an assay that detects random genomic off-target edits in mouse embryos, in which they co-expressed the editors with an sgRNA targeting the Tyrosinase gene. Compared to their traditional counterparts, the CE-CBEs generated significantly fewer single-nucleotide polymorphisms, and robust on-target editing. RNA-seq revealed that the CE-CBEs induced no off-target RNA editing.
Altogether, the results of this study demonstrate that Cas-embedding of base editing deaminases is a powerful strategy for reducing their off-target effects while retaining their on-target editing efficiency. Both CE-ABEs and CE-CBEs show dramatic increases in specificity, setting the stage for the use of these systems in the development of safer base editing therapies.
Cas-Embedded Deaminases Can Efficiently and Accurately Edit Clinically Relevant Targets
After the initial success in creating highly specific Cas-embedded base editors, a team of researchers from the University of Massachusetts Chan Medical School, Boston Children’s Hospital, Dana-Farber Cancer Institute, and Harvard Medical School sought to apply these systems to clinically relevant examples. Their next study, published in 2025 in Nucleic Acid Resources, demonstrates the power of CE-CBEs to perform therapeutic editing in primary human stem cells.
How delivery affects the off-target activity of base editors
CBE systems are typically delivered to cells either in plasmid format or as mRNA transcripts. Both of these modes result in the long-term expression of the base editor within cells, significantly increasing the risk of off-target editing.
In contrast, when they are delivered as a ribonucleoprotein (RNP) complex—in which the base editor is delivered in protein format alongside the sgRNA—they are capable of rapid, efficient editing activity, after which the base editing enzyme is quickly degraded by the host cell. This prevents further off-target editing without compromising on-target activity. Delivering the RNP via electroporation (direct delivery) also avoids the risk of random integration into the host cell genome associated with using plasmids.
CE-CBEs delivered by electroporation in RNP format would be ideal for creating safe and effective base editing therapies. However, producing high-quality, soluble CBE proteins for use in RNP formats has proven difficult. The team sought to overcome this obstacle by combining existing technologies to engineer CE-CBE systems for direct delivery in RNP format, producing high-yield, soluble, and stable proteins for therapeutic purposes.
Combining recent scientific advances to design soluble, stable CE-CBEs
The authors applied several recent technological advances to design their CE-CBE system for therapeutic editing. They employed the N57G variant of the single-domain cytidine deaminase APOBEC3A enzyme (eA3A), which has minimized off-target activity in DNA and RNA, and inserted it into the region of nCas9 that is tolerant of insertion using N- and C-terminal linkers.
Using AlphaFold protein modelling predictions, the team discovered that embedded eA3A would be able to access the target single-stranded DNA loop with ease using a very short linker of just two amino acids. However, because the optimal linker length was unknown and the editing activity of CBE systems linked to the N-terminus of nCas9 is affected by linker length, they created several different CE_CBEs with different linker lengths and tested them for activity and stability.
In these early tests, the editor with the longest linker lengths also displayed high editing efficiency (CE_16_eA3A). The team added nuclear localization signals (NLS) at the N- and C-termini of CE_16_eA3A, and fused a uracil glycosylase inhibitor (UGI) to the C-terminus to decrease unwanted substitutions. Electron microscopy revealed that the resulting protein was highly homogenous and did not aggregate when complexed with sgRNAs.
Compared to the eA3A system that was linked to the N-terminus of nCas9 (eA3A_BE3), CE_16_eA3A displayed enhanced expression in E. coli, resulting in double the yield of purified protein. When electroporated into HEK293T cells, the CE_16_eA3A system was more stable and resisted degradation by cellular proteases. Based on its enhanced characteristics, the team named the system soluble and stable Cas9-embedded CBE (sCE_CBE)
Delivering CE_CBEs to HEK293 cells in RNP format
To test the editing activity of sCE_CBE against eA3A_BE3, the authors targeted several sites in HEK293T cells using validating sgRNAs, delivering the system as an RNP complex via electroporation. High-throughput sequencing of the target sites showed that the sCE_CBE system resulted in significantly higher C-to-T editing efficiency for two of the three target sites, and comparable for the third.
To further characterise the CE-CBEs, the team analyzed the C-to-T editing activity and base conversion window of CE_16_eA3A and CE_2_eA3A, which had a shorter linker length, comparing them to eA3A_BE3 across nine targets and assessing bystander edits. The embedded editors showed improved efficiency and specificity, with the CE_16_eA3A system displaying minimal sgRNA-independent off-target editing.
The team also discovered that C-to-T editing activity is dose-dependent when CE-CBEs are electroporated into HEK293T cells as RNP complexes. Finally, low-level unwanted base substitutions were decreased, and product purity was increased by including the UGI and NLS as a recombinant purified protein within the RNP complex.
Editing a therapeutically relevant target in HSCs
The final test of the CE-CBEs was in a therapeutically relevant example: targeting the BCL11A erythroid enhancer region in hematopoietic stem and progenitor cells (HSPCs). Disruption of this target results in the production of fetal hemoglobin, ameliorating disease phenotypes in both sickle cell disease and beta-thalassemia.
Previous studies have shown that two electroporation cycles were needed to achieve high rates of editing, and this resulted in reduced cell viability, as well as lower chances of engraftment. However, the CE-CBEs were able to achieve high editing efficiencies of up to 86% with a single round of electroporation. Minimal cellular toxicity was observed, with no significant off-target editing or unwanted indels.
Importantly, this dose-dependent editing resulted in the rescue of disease phenotypes in the cells, which were successfully engrafted into a mouse model and displayed subsequent lineage differentiation.
CRISPR in the Clinic: What's Next?
Get the insights shaping today's clinical landscape and where opportunity is emerging.
The Power of AccuBase in the Clinic
With unprecedented precision, AccuBase is poised to overcome the obstacles that have previously prevented base editors from making significant strides in the clinic. Combining technological advances, this superior CBE system displays ideal stability, solubility, and specificity, achieving high editing efficiencies when delivered as an RNP complex via electroporation, and avoiding off-target and bystander editing.
This editing system is available as GMP-grade with high purity and yield, making it ideal for the development of base editing therapies. By eliminating the risks associated with double-stranded breaks and off-target editing, AccuBase provides the basis for safer, more effective cell and gene therapies for the treatment of genetic disease and more.
Ready to elevate your base editing workflow?
Explore the full product page, technical data, and ordering options.