The CRISPR field is moving fast! Don’t worry, we’ve got you covered. Check in every week for a quick summary of the biggest news and developments in genome engineering research so you can stay up to date with what’s happening in the world of CRISPR.
This week we report on a recently published study by researchers at Washington University School of Medicine in St. Louis. Their work investigates the use of CRISPR-Cas9 technology to build a new foundation for depression research.
Depression and the Serotonin Hypothesis
Depression (major depressive disorder or clinical depression) is a mood disorder that can severely impact the daily lives of patients. The most common symptoms are negative feelings that affect regular activities, such as sleeping, eating, and working. Depression is a complex disease, and a combination of factors likely play causal roles.
Serotonin, sometimes referred to as the “happy chemical” because it contributes to feelings of happiness, is produced by nerve cells and is thought to regulate mood and social behavior. Previously, researchers believed that reduced serotonin levels in the brain caused depression. The serotonin hypothesis of depression was first described in the 1960s.
As this hypothesis was the main driver for depression research, the majority of antidepressant drugs target serotonin receptors in the brain. Although a large percentage (13%) of Americans use antidepressants for various issues, a third of them fail to find relief with the existing options.
Targeting GABA Receptors to Tackle Depression
It is generally accepted that mood disorders such as depression, anxiety, schizophrenia, and bipolar disorder, are the result of a complex combination of genetic, biological, psychological, and environmental factors. Therefore, researchers are now investigating other mechanisms to unravel the underlying reasons for depression for developing effective therapeutics.
“There’s a real need to develop more effective antidepressants,” said Dr. Steven Mennerick, Professor of Psychiatry at Washington School of Medicine and lead author of the new study, in a press release. “The most commonly prescribed antidepressant drugs — such as Prozac, Paxil, and Zoloft — were approved by the FDA more than 30 years ago, and there’s been a dearth of new antidepressants since then. A completely new approach is warranted.”
Mennerick and team chose to focus on GABA receptors. These receptors are so named because they respond to the neurotransmitter gamma-aminobutyric acid (GABA), the primary inhibitory compound in the adult CNS. In other words, GABA is responsible for relaxation of brain and muscle; it prevents excitatory inputs from running amok.
Understanding the Different Types of GABA Receptors
GABA receptors, or more specifically GABAA type receptors, are involved in cellular signaling via direct interactions with GABA. There are two further subtypes of GABAA receptors: one containing a delta subunit (δ) and another type containing a gamma2 subunit (γ2).
It has previously been difficult to confidently assert the impact of neurosteroids on one particular subtype. Mennerick and his team suspected that specifically targeting neurosteroids to delta-type GABA receptors may hold the key for alleviating depressive symptoms.
“Neurosteroids are thought to selectively interact with delta-type receptors, and there’s evidence that those drugs may help patients suffering from depression,” said Mennerick in a press release.
Using CRISPR to Study Delta Subunit of GABAA Receptors
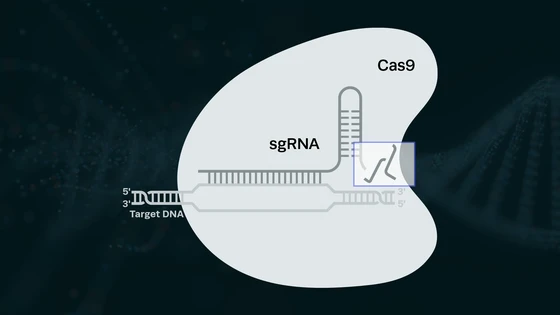
To specifically test the contribution of δ-containing receptors, Mennerick and his colleagues turned to CRISPR-Cas9 technology. They induced a point mutation in the gene encoding the GABAA δ subunit in the mouse hippocampus, which is the area of the brain involved in learning and memory. This rendered GABAA δ subunit resistant to picrotoxin, a chemical that blocks chloride channels to inhibit activity at the receptor.
The team then bred mice with these picrotoxin resistant GABAA δ receptors. After dissecting and slicing the hippocampus of developed mice, they stimulated neuronal cells in the presence of picrotoxin to note the specific response of the δ subunit. Previously, the δ subunit had been hypothesized to mainly participate in tonic (long term) response. However, these experiments revealed its contribution in phasic (short term) synaptic response as well after an electric stimulus.
The Future of GABA-Targeting for Depression
First author Dr. Min-Yu Sun, a postdoctoral researcher in Mennerick’s lab, explained that the research team plans to measure the actions of neurosteroids on various GABA receptor subtypes in the future.
“It’s very difficult to differentiate among different types of GABA receptors because they share so many common properties,” Sun said in a press release. “Previously, scientists really had no way to isolate the subtypes, but we can do that with CRISPR-Cas9 gene editing technology to learn how particular drugs affect individual receptor subtypes.”
Mennerick explained that if further studies confirmed that activating δ-containing receptors has antidepressant effects, the next step would be to develop and test more compounds that activate those receptors.
We must approach interpreting these results with caution given that the findings they have produced are so far only relevant to mice. That said, this study will hopefully build the foundation for research on different cell types and mechanistic actions in the future. Diversifying our approach to researching depression and moving away from the oversimplified serotonin hypothesis is an important step forward.
More CRISPR in the News…
In New CRISPR Tech: CRISPR-SKIP: Programmable gene splicing with single base editors
A new CRISPR-based technology has been unveiled this week. The new technique, developed by researchers at the University of Illinois, helps a cell’s internal machinery to “skip over” mutated portions of genes during the transcription process. The technique, termed CRISPR-SKIP, uses cytidine single-base DNA editor proteins to direct exon skipping by mutating specific DNA bases within splice acceptor sites.
CRISPR-Cas9 technologies typically “turn off” genes by creating a double-strand break in the DNA at the start of a target gene, causing mutations when the DNA break is repaired. CRISPR-SKIP does not break the DNA strands, it instead alters a single DNA base in the target sequence. Researchers hope that CRISPR-SKIP will offer a new approach to genome editing that avoids problems such as double-strand breaks in unintended places, and prevents the broken DNA from reattaching to different chromosomes. The researchers have tested CRISPR-SKIP in multiple cell lines from both mice and humans, both healthy and cancerous. The next step will be to test CRISPR-SKIP technology in vivo.
In Treating Silkworm Pathogens: CRISPR-Cas9 mediated disruption of the ie-0 and ie-2 as a therapeutic approach to BmNPV in transgenic Silkworm
Bombyx mori nuclear polyhedrosis virus (BmNPV) is an important pathogen of silkworms, but knowledge around its interaction with its host is limited. To effectively prevent and control pathogenic infections, a team of researchers from the University of Chongqing in China have performed gene editing and antiviral analysis of a transgenic silkworm line.
The team used CRISPR-Cas9 to engineer silkworm cell lines that are resistant to infection. Each year the silk industry suffers huge losses because of the disease caused by BmNPV – breeding resistant silkworm species could significantly reduce the economic losses experienced as a result.
In Oncogene-Induced Senescence: Functional CRISPR screen identifies AP1-associated enhancer regulating FOXF1 to modulate oncogene-induced senescence
Oncogene-induced senescence is a cellular mechanism that irreversibly prevents cells from multiplying. Bypassing this process is a critical step in the further development of cancerous tumors. It is hoped that by investigating and improving our understanding of this mechanism will lead to potential ways to trigger oncogene-induced senescence to prevent tumor growth. Researchers from The Netherlands have generated a CRISPR-Cas9 sgRNA library designed to target senescence-induced enhancers, and used it in a functional screen. The team identified a critical enhancer that they have named EnhAP1-OIS1 and determined the the FOXF1 gene is regulated by this enhancer. Their study demonstrates the power of CRISPR-based functional genomic screens and suggests specific enhancers that may hold potential as future therapeutic targets.
CRISPR 101 eBook
CRISPR has quickly become a standard laboratory tool for gene editing. As the adoption of CRISPR accelerates worldwide, up-to-date knowledge of the basics of CRISPR is essential for anyone in the field. From target identification studies to the recent breakthroughs in clinical trials, CRISPR is enabling scientists to unlock the power of the genome.
Download our CRISPR 101 eBook today to stay up to date on all your CRISPR basics and get the best results in your CRISPR experiments!