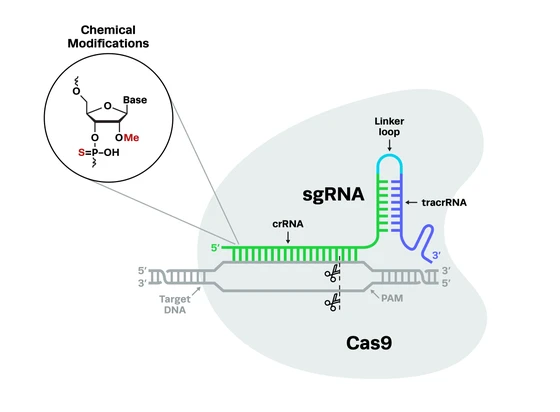
Contents
CRISPR-Cas9 gene-editing technology is now a well-known tool for the study and treatment of genetic diseases and cancer, but it’s also widely used in the field of infectious disease. CRISPR has allowed scientists to understand the biology and genetics of human pathogens, like viruses, bacteria, fungi, and parasites, and develop innovative tools for the diagnosis and treatment of these infections. In this blog, we’re shining a much-deserved spotlight on the use of CRISPR-Cas9 in infectious diseases.
CRISPR Research in Infectious Diseases
There are several different types of infectious diseases that can potentially be treated with CRISPR, including viruses, bacteria, and fungi. In this section, we’ll explore which diseases, caused by different pathogens, can potentially be treated and researched using CRISPR gene-editing technology, with examples for each.
CRISPR in viral diseases: COVID-19, HIV, and HPVViral pathogens, including DNA, RNA, and retroviruses, cause significant disease outbreaks in human populations. CRISPR has a range of applications in the study of viral pathogens and the development of new therapeutics, and the discovery of RNA-targeting Cas nucleases has opened new doors for targeting RNA viruses, including SARS-CoV-2.
Viral pathogens, including DNA, RNA, and retroviruses, cause significant disease outbreaks in human populations. CRISPR has a range of applications in the study of viral pathogens and the development of new therapeutics, and the discovery of RNA-targeting Cas nucleases has opened new doors for targeting RNA viruses, including SARS-CoV-2.
COVID-19CRISPR has been an important tool in the study of the current global pandemic of COVID-19, caused by the SARS-CoV-2 RNA virus. For instance, in a key collaborative study, Dr. Nevan Krogan and fellow UCSF researcher Dr. Jacqueline Fabius developed a host-pathogen protein-protein interaction map for SARS-CoV-2 and other coronaviruses. This interaction network identified hundreds of relevant proteins, then used high-throughput CRISPR gene knockout to functionally validate each of these. By pinpointing which factors had the most significant impact on infection and mapping these to available drugs, the study has led to further investigation of several potential treatments for COVID-19.
For more information on this and similar studies, you can listen to Dr. Krogan and Dr. Fabius discuss their research at the World CRISPR Day 2021 Disease Modeling & Research session.
CRISPR has been an important tool in the study of the current global pandemic of COVID-19, caused by the SARS-CoV-2 RNA virus. For instance, in a key collaborative study, Dr. Nevan Krogan and fellow UCSF researcher Dr. Jacqueline Fabius developed a host-pathogen protein-protein interaction map for SARS-CoV-2 and other coronaviruses. This interaction network identified hundreds of relevant proteins, then used high-throughput CRISPR gene knockout to functionally validate each of these. By pinpointing which factors had the most significant impact on infection and mapping these to available drugs, the study has led to further investigation of several potential treatments for COVID-19.
For more information on this and similar studies, you can listen to Dr. Krogan and Dr. Fabius discuss their research at the World CRISPR Day 2021 Disease Modeling & Research session.
CRISPR has massively accelerated both the search for novel drug targets for the treatment of infectious disease and the repurposing of existing drugs to treat emerging pathogens. For example, CRISPR knockout of a key enzyme in the fatty acid metabolism pathway, fatty acid synthase (FASN), demonstrated its role in SARS-CoV-2 infection and replication and suggested that existing inhibitors of FASN could prevent infection.
Another recent study used CRISPR knockouts to identify genes in human host cells that restrict replication of SARS-CoV-2. These functional CRISPR screens elucidated the role of death-domain associated protein 6 (DAXX), a scaffold protein that had previously been described to limit the replication of DNA viruses and retroviruses, but not RNA viruses like SARS-CoV-2. Interestingly, the study revealed that SARS-CoV-2 had evolved the ability to degrade DAXX, but this effect could be prevented by treatment with GRL-0617.
There have been some limitations to the direct use of CRISPR to treat RNA viruses, like SARS-CoV-2 in human patients. Cas13, an RNA-targeting nuclease, displayed substantial collateral activity and destroyed all transcripts within cells after cutting the target. However, after the recent discovery of Cas7-11, an RNA-targeting nuclease that displays no collateral activity, there has been renewed interest in the therapeutic use of RNA-editing CRISPR systems in humans.
HPVHuman papillomavirus (HPV) is a small DNA virus that is known to cause cervical and other cancers. Research has identified the key genes of HPV responsible for oncogenesis—E6 and E7—leading to these genes being targeted with CRISPR as a potential therapeutic tool for HPV. For example, several studies have confirmed that targeting the E6 and E7 genes with CRISPR-Cas9 leads to decreased levels of the proteins, apoptosis of infected cells, and growth inhibition.
Pre-clinical studies used stealth liposomes to deliver CRISPR-Cas9 editing components to target the E7 gene in HPV16 tumors in mice. The treatment effectively cleared the tumors without causing toxicity to the liver or spleen of the mice. Positive results like these led to clinical trials of this therapy being tested in human patients.
Human papillomavirus (HPV) is a small DNA virus that is known to cause cervical and other cancers. Research has identified the key genes of HPV responsible for oncogenesis—E6 and E7—leading to these genes being targeted with CRISPR as a potential therapeutic tool for HPV. For example, several studies have confirmed that targeting the E6 and E7 genes with CRISPR-Cas9 leads to decreased levels of the proteins, apoptosis of infected cells, and growth inhibition.
Pre-clinical studies used stealth liposomes to deliver CRISPR-Cas9 editing components to target the E7 gene in HPV16 tumors in mice. The treatment effectively cleared the tumors without causing toxicity to the liver or spleen of the mice. Positive results like these led to clinical trials of this therapy being tested in human patients.
HIVRetroviruses like human immunodeficiency virus (HIV) are a specific type of RNA virus that cleverly integrate their genetic material into host genomes and are therefore notoriously difficult to treat. This is another research field in which CRISPR has provided new hope. By targeting retroviruses, CRISPR can be used to excise the viral DNA from the genome of host cells.
The FDA recently gave the green light to Excision BioTherapeutics for a clinical trial of EBT-101, a one-time CRISPR-based treatment to cut HIV from human cells. You can listen to Dr. TJ Cradick, CSO of Excision, discuss the EBT-101 therapy for HIV excision in his presentation at World CRISPR Day 2021.
Retroviruses like human immunodeficiency virus (HIV) are a specific type of RNA virus that cleverly integrate their genetic material into host genomes and are therefore notoriously difficult to treat. This is another research field in which CRISPR has provided new hope. By targeting retroviruses, CRISPR can be used to excise the viral DNA from the genome of host cells.
The FDA recently gave the green light to Excision BioTherapeutics for a clinical trial of EBT-101, a one-time CRISPR-based treatment to cut HIV from human cells. You can listen to Dr. TJ Cradick, CSO of Excision, discuss the EBT-101 therapy for HIV excision in his presentation at World CRISPR Day 2021.
Each virus has different modes of entry into human host cells; narrowing down the search for the gene/s responsible for infection is like trying to find a needle in a haystack without the correct tools. This is another area in which CRISPR has become increasingly relevant, due to the ease and scalability of functional genomics studies using CRISPR knockouts. A 2018 study from Dr. Nevan Krogan’s lab at UCSF developed a high-throughput CRISPR screen in primary human CD4+ T cells to identify factors involved in HIV infection and pathogenesis.
Targeting pathogenic bacteria using CRISPR CRISPR-Cas9 is part of bacterial immune systems, used to protect bacteria from invading viruses called bacteriophage. Ironically, CRISPR is now being used to do the reverse - engineering bacteriophages to be better at attacking and killing pathogenic bacteria. One of the main proponents of this novel therapy is Locus Biosciences, a clinical-stage biotech company.
Locus Bio recently completed a Phase1b clinical trial using CRISPR-engineered bacteriophage (crPhage) to target the pathogenic Escherichia coli bacteria that cause urinary tract infections (UTIs). By the addition of Cas3 and a specific guide RNA sequence, the crPhage destroys the E.coli genome without affecting any beneficial bacteria or human cells.
Other studies seeking to deliver CRISPR components to selectively target pathogenic bacteria have utilized phagemids, which have dual plasmid and phage characteristics, or conjugative plasmids, which can confer antibiotic resistance between bacteria through horizontal gene transfer. For example, a 2019 study used E. coli conjugative plasmids to deliver CRISPR components and selectively target Salmonella enterica.
CRISPR-Cas9 is part of bacterial immune systems, used to protect bacteria from invading viruses called bacteriophage. Ironically, CRISPR is now being used to do the reverse - engineering bacteriophages to be better at attacking and killing pathogenic bacteria. One of the main proponents of this novel therapy is Locus Biosciences, a clinical-stage biotech company.
Locus Bio recently completed a Phase1b clinical trial using CRISPR-engineered bacteriophage (crPhage) to target the pathogenic Escherichia coli bacteria that cause urinary tract infections (UTIs). By the addition of Cas3 and a specific guide RNA sequence, the crPhage destroys the E.coli genome without affecting any beneficial bacteria or human cells.
Other studies seeking to deliver CRISPR components to selectively target pathogenic bacteria have utilized phagemids, which have dual plasmid and phage characteristics, or conjugative plasmids, which can confer antibiotic resistance between bacteria through horizontal gene transfer. For example, a 2019 study used E. coli conjugative plasmids to deliver CRISPR components and selectively target Salmonella enterica.
Gene editing in fungal infectious diseasesAlthough we generally hear more about viruses and bacteria, fungal pathogens are also a threat to human health, causing 1.6 million deaths per year globally. There are also growing concerns about the prevalence of antifungal-resistant species. Functional genomics studies using gene-editing technology allow us to understand the biology of fungi, revealing mechanisms for drug resistance and contributing to the development of novel therapeutics.
A key challenge in the study of fungi and the production of new treatments for fungal pathogens is that traditional genetic manipulation has proven difficult in many fungal species. This is due to several unique characteristics of fungi, including low efficiencies of homology-directed repair (HDR) in fungal cells, codon bias, and complex genomes and reproductive cycles. CRISPR systems have created new avenues for the genetic manipulation of fungi, however, and there is exciting research being done in this area.
For example, a 2016 study developed an efficient CRISPR mutagenesis strategy utilizing microhomology-mediated end-joining (MMEJ) in the pathogen Aspergillus fumigatus. Base editors have also been suggested as a tool to efficiently edit fungal genomes because they do not induce double-stranded breaks (DSBs). CRISPR-Cas9 has also been used to generate gene drive platforms in the common fungus Candida albicans. While current methods for gene editing in fungi are predominantly used for research purposes, they may also have the future potential to treat fungal infections directly.
Check out our podcast interview with Dr. Rebecca Shapiro where we discuss the identification of disease-causing mechanisms, engineering gene drives, and treatment of fungal infections.
Although we generally hear more about viruses and bacteria, fungal pathogens are also a threat to human health, causing 1.6 million deaths per year globally. There are also growing concerns about the prevalence of antifungal-resistant species. Functional genomics studies using gene-editing technology allow us to understand the biology of fungi, revealing mechanisms for drug resistance and contributing to the development of novel therapeutics.
A key challenge in the study of fungi and the production of new treatments for fungal pathogens is that traditional genetic manipulation has proven difficult in many fungal species. This is due to several unique characteristics of fungi, including low efficiencies of homology-directed repair (HDR) in fungal cells, codon bias, and complex genomes and reproductive cycles. CRISPR systems have created new avenues for the genetic manipulation of fungi, however, and there is exciting research being done in this area.
For example, a 2016 study developed an efficient CRISPR mutagenesis strategy utilizing microhomology-mediated end-joining (MMEJ) in the pathogen Aspergillus fumigatus. Base editors have also been suggested as a tool to efficiently edit fungal genomes because they do not induce double-stranded breaks (DSBs). CRISPR-Cas9 has also been used to generate gene drive platforms in the common fungus Candida albicans. While current methods for gene editing in fungi are predominantly used for research purposes, they may also have the future potential to treat fungal infections directly.
Check out our podcast interview with Dr. Rebecca Shapiro where we discuss the identification of disease-causing mechanisms, engineering gene drives, and treatment of fungal infections.
CRISPR in Fungi: An Interview with Rebecca Shapiro
Rebecca Shapiro, Assistant Professor at the University of Guelph, talks about CRISPR genome editing in fungal pathogens to understand their disease-causing mechanism. Her lab also works on gene drives in fungi for studying large scale genetic interactions and developing ways for treating fungal biofilms.

CRISPR Diagnostics for Infectious Disease
In the last few years, CRISPR has been widely used for diagnostic applications, using the power of Cas9 and other Cas nucleases as search tools, not for their ability to create double-stranded breaks. Before the COVID-19 pandemic, the field of CRISPR diagnostics was in its infancy. Two years into the pandemic, there has been a massive explosion in this area of research. With the variety of Cas nucleases available, including both DNA- and RNA-targeting nucleases, there seems to be a potential CRISPR diagnostic for every type of virus.
For SARS-CoV-2, which is an RNA virus, RNA-targeting Cas13 and its variants are the main nucleases currently being used. CRISPR diagnostics for SARS-CoV-2 are just as accurate as traditional PCR-based tests and have the additional advantage of speed; while PCR tests take several hours, CRISPR-based methods can produce results in as little as 20 minutes. Mammoth Biosciences and Sherlock Biosciences are the leading proponents of CRISPR diagnostics for COVID-19. Cardea Bio is a prominent company working in the CRISPR diagnostics space for pathogen and infectious disease testing.
Coronavirus Detection Using CRISPR Diagnostics
As the fight against coronavirus disease intensifies, CRISPR is at the forefront of this battle. Recently, Mammoth Biosciences and Feng Zhang’s SHERLOCK team have independently reported CRISPR-based platforms for sensitive detection of viral RNA.
While diagnostics for COVID-19 certainly get the most attention, there are many other pathogens that can be accurately and rapidly diagnosed using CRISPR technology. This includes other RNA viruses such as Ebola virus, Zika virus, and Dengue virus, and DNA viruses including HPV, CMV, EBV, and BKV.
CRISPR diagnostics have also been developed for bacterial pathogens, including Salmonella enteritidis, Mycobacterium tuberculosis, Pseudomonas aeruginosa, Staphylococcus aureus, and Listeria monocytogenes, and even for parasites causing malaria and other Plasmodium species. Read more about CRISPR diagnostics for infectious disease in this excellent 2021 review.
CRISPR Cell Therapies for Infectious Disease
CRISPR-edited cell therapies have been gaining momentum in recent years due to their massive potential in treating genetic diseases and cancer. However, these novel therapies can also be used to effectively treat infectious diseases. Here, we’ll take a look at CRISPR gene-editing in T cells and B cells, and how they can be used to combat a variety of pathogens.
T cell biobanks for treatment of viral infectionsViral infections are common in cancer patients who have received hematopoietic stem cell transplants, and they are often life-threatening. A proven treatment for these infections is to administer virus-specific T cells (VSTs), however, the VSTs are unable to persist in patients who have been treated with immunosuppressive glucocorticoid therapy. A recent study used CRISPR to overcome this problem, generating VSTs at scale with the glucocorticoid receptor gene knocked out.
Without the receptor responsible for binding glucocorticoids, the VSTs are able to avoid apoptosis and treat viral infections. The same approach has been applied to the treatment of COVID-19, generating a biobank of GR-knockout T cells specific to the SARS-CoV-2 virus as an off-the-shelf treatment for COVID-19.
For more information on CRISPR T cell therapy for viral infections, you can watch Dr. May Daher’s World CRISPR Day 2021 talk about her research at the MD Anderson Cancer Center.
Viral infections are common in cancer patients who have received hematopoietic stem cell transplants, and they are often life-threatening. A proven treatment for these infections is to administer virus-specific T cells (VSTs), however, the VSTs are unable to persist in patients who have been treated with immunosuppressive glucocorticoid therapy. A recent study used CRISPR to overcome this problem, generating VSTs at scale with the glucocorticoid receptor gene knocked out.
Without the receptor responsible for binding glucocorticoids, the VSTs are able to avoid apoptosis and treat viral infections. The same approach has been applied to the treatment of COVID-19, generating a biobank of GR-knockout T cells specific to the SARS-CoV-2 virus as an off-the-shelf treatment for COVID-19.
For more information on CRISPR T cell therapy for viral infections, you can watch Dr. May Daher’s World CRISPR Day 2021 talk about her research at the MD Anderson Cancer Center.
CRISPR-edited B cells: An alternative to vaccines and antibody therapy?Another nascent application of CRISPR technology is gene-edited B cell therapy. B cells are a central component of the adaptive immune system, producing antibodies to help fight off pathogens. Vaccines are administered with the expectation that B cells will be stimulated to produce the correct antibodies, priming the immune system for infection with the real pathogen. Vaccines are not available for all pathogenic viruses, however, and they are not always effective in all patient groups.
CRISPR-edited B cell therapy has been proposed as a vaccine alternative. By performing targeted CRISPR knock-in of the necessary genes, B cells collected from peripheral blood can be used to generate large amounts of neutralizing antibodies against common viral pathogens. A 2019 study took this approach in order to treat respiratory syncytial virus (RSV) in a mouse model and also proved CRISPR-edited B cells can produce neutralizing antibodies against HIV, EBV, and influenza.
Another nascent application of CRISPR technology is gene-edited B cell therapy. B cells are a central component of the adaptive immune system, producing antibodies to help fight off pathogens. Vaccines are administered with the expectation that B cells will be stimulated to produce the correct antibodies, priming the immune system for infection with the real pathogen. Vaccines are not available for all pathogenic viruses, however, and they are not always effective in all patient groups.
CRISPR-edited B cell therapy has been proposed as a vaccine alternative. By performing targeted CRISPR knock-in of the necessary genes, B cells collected from peripheral blood can be used to generate large amounts of neutralizing antibodies against common viral pathogens. A 2019 study took this approach in order to treat respiratory syncytial virus (RSV) in a mouse model and also proved CRISPR-edited B cells can produce neutralizing antibodies against HIV, EBV, and influenza.
CRISPR Gene Drives to Eliminate Disease Vectors
Many human pathogens are vector-borne, meaning that they are transmitted from blood-feeding arthropods like mosquitos, ticks, and fleas. This includes malaria, Zika virus, Lyme disease, tick-borne encephalitis, West Nile virus, and yellow fever, to name just a few. Other pathogens are transmitted to humans via birds, snails, rats, or other mammals. With gene-editing technology, however, the opportunity now exists to control or eliminate the spread of these animal vectors, thus preventing the transmission of the pathogens they carry.
Gene drives, self-propagating genetic elements which spread through populations, have been a hot topic in the field of biological control in recent years. A key example of a gene drive using CRISPR technology can be seen in the malaria-transmitting Anopheles mosquito. Scientists have genetically engineered the species, altering the sex-determination gene to make males more dominant than females. This change results in the eradication of female mosquitos, preventing the spread of the disease through blood-feeding.
Read more about gene drives in Anopheles mosquitos in our blog below.
Eradicating Malaria with CRISPR: Mosquito Gene Editing Approach
Malaria affects millions of people globally every year. No longer confined to tropical countries, outbreaks of malaria are becoming increasingly common in the United States. With the need for an effective way to combat the disease, scientists are now exploring the use of CRISPR technology to genetically modify mosquitoes and control the spread of malaria.

Is CRISPR the Solution to Antibiotic Resistance?
Currently, most bacterial infections are treated using antibiotic drugs. Many of these are broad-spectrum antibiotics, meaning that they will kill a range of bacterial species, including beneficial bacteria, which can in turn lead to other health issues. The other problem with the widespread use of antibiotics is the rise of antibiotic-resistant ‘superbugs’. With a paucity of new antibiotics being identified and commercialized, options are running out for the treatment of these resistant microbes.
CRISPR has not only enabled the identification of novel drug candidates but it can actually be used to identify antimicrobial resistance (AMR) genes. It can also be used as a therapeutic tool to directly treat antibiotic-resistant bacteria and other pathogens. By using a species-specific guide RNA sequence, the CRISPR system can be directed to cut and destroy only pathogenic bacteria, leaving commensal and beneficial microbes unscathed.
Preventing the Next Global Pandemic with CRISPR
CRISPR gene-editing technology could help prevent future global pandemics via several different pathways. With CRISPR-based diagnostic tools for rapid, point-of-care testing, emerging disease outbreaks could be monitored much more efficiently and in real-time, removing the bottlenecks associated with traditional testing procedures.
The technology is also being increasingly applied in vaccine research and development, as described in a recent review. With the more widespread use of CRISPR antimicrobials for the treatment of pathogens, the rise of antimicrobial resistance could also be significantly impeded, preventing the spread of hard-to-treat ‘superbugs’.
Other nascent applications of CRISPR in infectious disease prevention involve engineering animals which are known natural reservoirs of disease. For example, Tropic Biosciences have engineered poultry to be resistant to various strains of avian influenza virus, spillover of which can cause fatal disease in humans. Cows have been similarly engineered for resistance to the bacteria which causes tuberculosis (TB).
More recently, it has been suggested that bats, which are natural reservoirs of coronaviruses like SARS-CoV-2, should also be genetically engineered. By creating gene drives to spread disease resistance among bat populations, zoonotic transmission of these pathogens could be avoided, eliminating new outbreaks before they begin.
We hope this blog helped you understand more about the role of CRISPR gene-editing technology in the field of infectious disease, from diagnosis to treatment. With so many applications across a range of human pathogens, we’re sure this will be an exciting area of research for years to come.