The IP framework for CRISPR-based therapies is a dynamic and evolving landscape. Foundational patents are primarily owned by institutions like the University of California, Berkeley and the Broad Institute, among others. These institutions hold the rights to pivotal technologies like CRISPR-Cas9, shaping how licensing agreements are managed. The dispute over which institute first discovered and developed technologies like CRISPR-Cas9 has prompted legal battles, influencing licensing terms globally.
With these foundational patents in place, the market is driven by licenses and sub-licenses granted to organizations looking to develop therapies. These organizations must assess whether a license is available and economically feasible to support freedom-to-operate, a hurdle that can define or derail early strategies.
Challenges Surrounding IP in CRISPR-Based Therapies
The volume of IP surrounding CRISPR-based therapies creates freedom-to-operate complexities that organizations must manage:
Patent Thickets
Overlapping patents create intricate webs of ownership that complicate how organizations utilize CRISPR for therapeutics. Navigating these "patent thickets" can lead to delays, increased legal costs, and uncertain outcomes when planning therapeutic pipelines. Companies must often dedicate significant resources to legal and strategic planning to ensure they avoid infringement, impacting timelines and budgets. This complexity underscores the need for streamlined licensing or innovative IP strategies to move forward efficiently.
High Licensing Costs
Expenses associated with obtaining licenses for CRISPR-based technologies can pose significant financial hurdles, particularly for smaller biotech organizations with limited budgets. These costs can exceed initial projections, forcing companies to prioritize certain projects over others or seek additional funding. For researchers at smaller institutions or early-stage companies, high licensing fees may block access to cutting-edge tools, limiting their ability to compete with larger organizations. Reducing financial barriers and creating more accessible cost structures can empower smaller biotech organizations to remain competitive and innovate freely.
Access Barriers
Exclusive licensing agreements can limit access to foundational CRISPR tools for smaller organizations, independent researchers, and biotech startups. Without access to these foundational technologies, many promising research initiatives struggle to gain traction. For smaller organizations, competing in a field dominated by companies with exclusive rights or an abundance of resources often feels like an uphill battle. By prioritizing partnerships and inclusive licensing models, organizations can expand access and foster equitable innovation across the industry.
Uncertainty from Legal Disputes over IP
Ongoing patent disputes create an unpredictable environment for investors and developers, hampering long-term planning and stalling progress. Legal disputes may result in changes to licensing rights or raise uncertainty about continued access to key technologies, affecting aspects of therapy development, from preclinical optimization to commercialization. For organizations, this uncertainty can discourage investment and delay vital research, adding pressure to operate carefully while planning for contingencies. Clear resolution processes and robust IP strategies are essential to mitigate such risks and sustain momentum in the field.
Strategies to Overcome IP Challenges
To mitigate the complexities of IP in the CRISPR landscape, organizations have employed various approaches:
Sublicensing Agreements
Sublicensing CRISPR tools can help organizations gain legal access to patented CRISPR technologies, supporting compliance while potentially accelerating the therapeutic timeline. By sublicensing foundational CRISPR technologies, researchers can operate in the licensed field of use without infringing primary IP holders' patents. This method, by granting rights to patented IP to more parties, promotes collaboration and expands opportunities for innovation. However, these agreements can come with significant costs, especially for smaller biotech organizations operating on constrained budgets. Additionally, they may limit competitive advantages by restricting exclusivity over certain gene targets or therapeutic applications, making it critical for organizations to weigh the trade-offs carefully.
Partnerships with Institutional Inventors
Collaborating with academic institutions or patent holders makes it possible for biotech and pharma organizations to secure access to groundbreaking technologies. These partnerships often serve as a catalyst for long-term innovation, providing researchers with the freedom to explore novel gene therapies while navigating the patent landscape more efficiently. On the downside, establishing such partnerships can be complicated, requiring lengthy negotiations that might delay critical projects. Furthermore, the dependence on institutional partners can introduce risks, as funding shifts or changes in priorities could impact the partnership’s stability and progress.
Development of Alternative Nucleases
Another way organizations have tackled the constraints of working around foundational CRISPR IP is by designing or identifying alternative nucleases that fall outside of the scope of these foundational patents. For instance, innovative options such as hfCas12Max and eSpOT-ON have broadened the toolbox for researchers, offering new functionality outside of the foundational IP claims. Sub-licensing these novel nucleases further streamlines access, allowing researchers and developers to leverage cutting-edge tools. This approach may result in reduced upfront costs and also potentially accelerate the integration of these technologies into existing workflows, making it a practical and scalable solution for many organizations. By sub-licensing these novel nucleases, teams can gain access to powerful resources that expand therapeutic possibilities.
Patent Pooling
While not yet common in the CRISPR space, patent pooling could simplify access to foundational IP by allowing multiple parties to license overlapping patents within a single agreement. This strategy reduces negotiation overhead, fosters collaboration, and lowers costs compared to negotiating individual licenses. By centralizing access through collective agreements, organizations can build research pipelines more quickly and without unnecessary conflicts. However, this strategy can face challenges if major patent holders choose not to participate, leaving gaps in the pool’s utility. Smaller organizations may also struggle if fees associated with the pool remain high, potentially offsetting the benefits of this otherwise inclusive solution.
Interested in testing eSpOT-ON protein?
Schedule a call with our sales team to learn how you can get started. Just mention "try out eSpOT-ON Protein" to explore your options with them.
Benefits of Leveraging Novel Nucleases
The emergence of novel nucleases has fostered innovation and transformed the IP landscape, offering numerous advantages that may alleviate the freedom-to-operate challenges around foundational CRISPR patents.
Improved Licensing Models Enhance Economic Viability
One of the most significant benefits novel nucleases offer is the potential for improved licensing terms. Developing traditional therapies often come with economic barriers due to complicated and costly licensing agreements. However, the emergence of novel nucleases enables flexible and favorable licensing terms, improving the economics of therapeutic development. Synthego’s sublicensing approach enables potential sublicensees to identify and optimize their CRISPR-based editing strategies through gene target and nuclease selection before committing to a license. With more nucleases coming to market, competition drives simpler financial terms for licensing and reduces negotiation complexities between parties.
Past Therapies Become Economically Viable
Advancements in nucleases and licensing models have revitalized therapies that were once abandoned due to high costs or poor return on investment potential. Enhanced economic feasibility opens doors for reconsidering vital therapies that address unmet medical needs. This shift opens the door for a broader range of therapeutic innovations, extending the reach and impact of CRISPR technology.
Expanding Access to Research Opportunities with Sub-Licenses
Obtaining a sub-license provides the sublicensee with access to defined IP rights that support freedom-to-operate and enables IP protection while supporting continuous innovation. This may include the rights to specific gene targets or applications, enabling researchers to conduct their studies confidently without interruptions. It ensures that intellectual property remains safeguarded while advancing through the therapeutic continuum. This model fosters innovation while protecting the development of breakthrough therapies.
This protection is particularly crucial for long-term projects requiring substantial investment and development time. For projects requiring years of development, early integration of sub-licenses creates a stable foundation for financial and operational success. Establishing this protection early helps prevent future economic volatility in the market value of high-performing nucleases and gives organizations a more predictable financial path as therapies are developed.
Access to Previously Restricted Gene Targets
Novel advanced nucleases may enable researchers and developers access to gene targets that were previously out of reach. Each nuclease has a unique PAM sequence recognition profile that enables it to cut at specific sites in the genome, providing a fresh evaluation of approaches that might not have been possible with earlier nucleases. This exclusivity is a game-changer for multiple reasons.
Having access to new gene targets allows researchers to explore and develop novel therapies that address unmet medical needs. This access fosters innovation and strengthens the competitive positioning of organizations by allowing them to offer unique treatments. From a business standpoint, licensing in IP rights enables a strategic competitive advantage by differentiating companies in an increasingly crowded cell and gene therapy market.
Strategic Competitive Advantage
Possessing a license to novel CRISPR IP rights positions organizations as market leaders in the rapidly evolving cell and gene therapy space. By strategically gaining access to novel CRISPR tools, organizations can differentiate themselves from competitors while gaining an edge in securing partnerships and investments. From a business perspective, this creates a favorable foundation for scaling operations and entering new markets.Synthego offers novel products, including hfCas12Max, eSpOT-ON, and AccuBaseTM nuclease, under simplified, accessible licensing terms that minimize financial and legal barriers. By lowering upfront costs and minimizing licensing complexities, we enable organizations of all sizes to access advanced CRISPR tools in their development pipelines. This approach helps both large organizations and small biotech startups focus on developing therapies by reducing several common licensing hurdles.
Synthego’s Role in Addressing IP Challenges
Synthego is at the forefront of solving IP-related challenges for CRISPR-based therapeutic developers. Synthego's comprehensive approach ensures researchers, scientists, and biotech organizations have the solutions and support needed to address IP challenges effectively. By providing streamlined access to cutting-edge nucleases with the aim of simplifying licensing, enhancing flexibility, and empowering discovery, Synthego is reducing barriers to gene editing by allowing researchers and developers to overcome IP complexities with ease.
Simplified Licensing Models
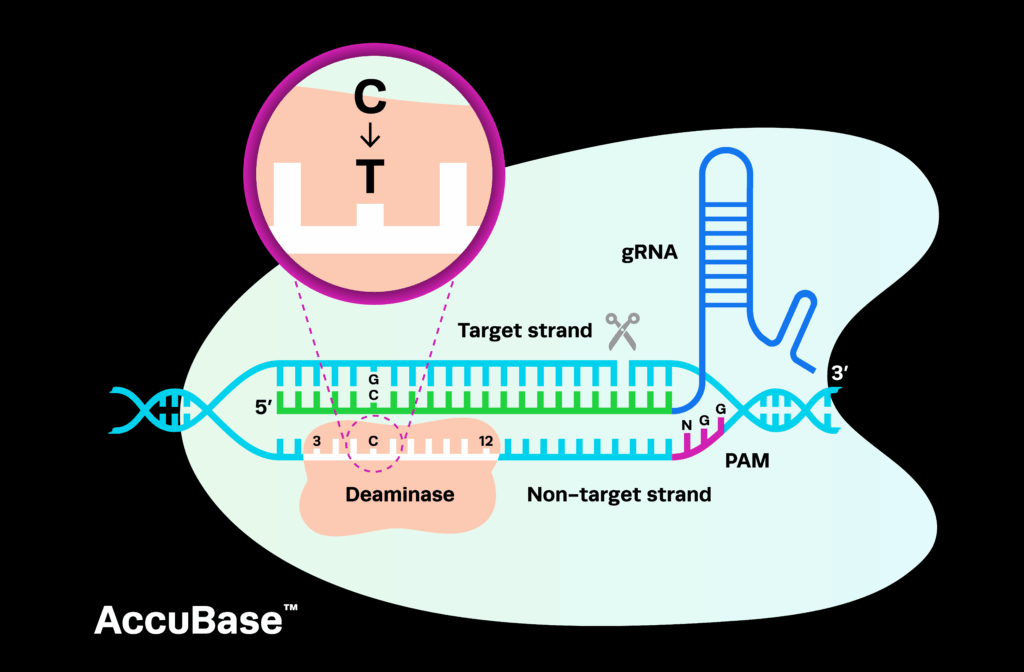
Synthego offers novel products, including hfCas12Max, eSpOT-ON, and AccuBaseTM nuclease, under simplified, accessible licensing terms that minimize financial and legal barriers. By lowering upfront costs and minimizing licensing complexities, we enable organizations of all sizes to access advanced CRISPR tools in their development pipelines. This approach helps both large organizations and small biotech startups focus on developing therapies by reducing several common licensing hurdles.
Synthego's CRISPR Nucleases
With a successful track record in synthesizing and optimizing gRNAs, Synthego is dedicated to equipping you with high-performance nucleases precisely paired with engineered gRNAs to meet your most challenging CRISPR objectives.

Flexible Access
Synthego prioritizes flexibility by offering RUO solutions that seamlessly integrate into research workflows before entering into licensing commitments. This “try-before-you-buy” model ensures researchers can confidently evaluate and optimize their CRISPR strategies using tools like hfCas12Max and eSpOT-ON, allowing for informed decision-making as projects progress. By providing this level of accessibility, we help customers avoid wasted investment and mitigate early uncertainty, giving them the freedom to focus on producing impactful scientific results.
Comprehensive Support
Beyond providing advanced gene editing solutions, Synthego backs our customers with unmatched technical and strategic guidance. Whether it’s troubleshooting experimental challenges, advising on nuclease selection, or assisting with regulatory compliance, our team of experts is committed to maximizing customer success. This hands-on approach ensures that scientists, developers, and institutional partners are equipped to tackle IP concerns confidently while keeping their research goals on track.
Timing of IP Considerations in Therapy Development
IP considerations are pivotal at multiple points. Ideally, developers address IP as early as possible during nuclease and gene target selection. Early planning avoids wasted investment in tools that prove inaccessible later. By securing IP rights at the earliest stages, organizations can approach therapy design and investor discussions with greater confidence. IP considerations play a critical role in preclinical study design and optimization. Key areas impacted include:
Tool Selection
Exclusive intellectual property rights directly influence which CRISPR tools and nucleases are legally usable in a study, often limiting access to certain advanced technologies. For researchers, selecting a nuclease that aligns with IP rights is more than just a legal formality – it is a strategic decision that can define the efficiency and scope of their experiments. Simplified sublicensing models, such as those offered by Synthego, allow scientists to focus on innovation rather than legal hurdles, empowering teams to streamline their planning process.
Target Validation
Preclinical investigation is often limited to gene targets covered by available nuclease licenses, forcing researchers to pivot from their initial objectives, reshaping experiments to comply with what is legally accessible. For biotech organizations, this may add an additional layer of planning and increase the cost of validating targets. By securing flexible licensing options or working with providers who simplify these restrictions, research teams can validate targets more efficiently, ensuring their preclinical work remains aligned with their overall therapeutic goals.
Design Planning
The structure and execution of preclinical studies must comply with IP rights to avoid legal complications when transitioning to clinical stages. This alignment requires teams to consider IP implications from the outset of their study design, from choosing editing tools to mapping out their workflows. For biotech organizations, this can involve working closely with legal and business development teams to ensure compliance while maximizing research output. Partnering with flexible IP-focused providers can minimize potential barriers, allowing researchers to focus on innovation and seamless progression through the therapeutic development pipeline.
Featured Products from Synthego
hfCas12Max
hfCas12Max is a next-generation nuclease that expands the possibilities for precise gene editing with versatile PAM recognition, allowing researchers to target previously inaccessible regions of the genome and ensure more reliable editing. Synthego offers a simplified sublicensing model for hfCas12Max that provides researchers enhanced freedom to explore challenging gene targets.
Crucially, hfCas12Max addresses the IP challenges that often hinder genetic innovation. Unlike traditional CRISPR technologies tied to extensive patent disputes, it may reduce exposure to heavily patented systems, thereby offering a streamlined licensing model. This may reduce licensing costs and administrative barriers, enabling researchers and biotech companies to innovate with greater flexibility. Its combination of advanced capabilities and licensing flexibility makes hfCas12Max an essential tool for driving the future of gene editing breakthroughs.
High Fidelity Cas12 CRISPR Nuclease
hfCas12Max nuclease is a novel, Cas12 variant engineered for therapeutic application because of its broad PAM recognition profile that maintains its high fidelity, outperforming other Cas12 nucleases.
eSpOT-ON: High Fidelity Nuclease
Optimized for efficiency and specificity, eSpOT-ON sets new standards for reliable gene editing, offering high on-target activity, low off-target effects, and a significantly reduced risk of chromosomal translocations. But just as important is how easily you can access and use this technology. Unlike Cas9 nucleases tied to complex and costly licensing structures, eSpOT-ON is available through a simplified, developer-friendly sub-licensing model.
Our approach removes traditional bottlenecks that delay innovation. With lower upfront costs, clear, milestone-based terms, and straightforward agreements, eSpOT-ON allows researchers to focus on advancing their therapeutic programs. Whether starting preclinical studies or moving into the clinic, this flexible licensing model is designed to scale with your development and support a faster path to market.
Interested in testing eSpOT-ON protein?
Case Study: Vita Therapeutics and hfCas12Max
A prime example of Synthego’s impact can be seen in Vita Therapeutics’ decision to sublicense hfCas12Max. By integrating this advanced nuclease into their pipeline, Vita is developing stronger therapeutic platforms with improved editing efficiencies. “With encouraging early performance data, Vita Therapeutics plans to leverage hfCas12Max to develop hypoimmunogenic master cell banks (MCBs), which will serve as a critical platform for advancing our iPSC-based therapies for neuromuscular disorders. We’re excited about this partnership and the impact it can have on the patients we’re hoping to treat” said Douglas Falk, CEO of Vita Therapeutics.
This partnership showcases how innovative licensing models can support cutting-edge research while driving the creation of novel treatments.
Unlocking the Future of CRISPR-Based Therapies
The integration of novel nucleases into CRISPR therapies offers a myriad of benefits from an IP standpoint. Improved licensing structures make previously unviable therapies economically feasible and provide access to critical gene targets. By addressing the challenges of the current IP landscape and offering innovative solutions, Synthego is empowering scientists, biotech investors, and organizations to unlock new arenas of innovation.
Whether you’re navigating IP challenges for the first time or looking to optimize your CRISPR-based therapeutic pipeline, understanding and leveraging IP strategies is crucial. With tools like hfCas12Max and eSpOT-ON nucleases, Synthego is enabling a future where scientific breakthroughs are no longer defined by IP obstacles but supported by them.
Are You Looking For A Simplified Path To Market For Your Therapy?
Talk to us about your work and our available nuclease sub-licensing options.